Editor’s Note: Nathan Wolfe is Founder & CEO of Metabiota, a company addressing global infectious disease risk, & the Lorry I. Lokey Consulting Professor of Human Biology at Stanford University. Follow him on Twitter: @virushunter. Oliver Pybus is a Professor at the University of Oxford and a Consulting Senior Scientist at Metabiota and a co-author of a new study in Nature that reports the discovery of the origins of the H7N9 influenza virus.
Story highlights
Authors: New study traces origin of flu virus that kills a third of victims
They say it shows the need for vigilance and prevention measures vs. flu
Ducks could help create the next flu pandemic, need careful monitoring, they say
Authors: There's a growing arsenal of tools that will be useful in prevention
Influenza is a little like poker. Only when it wins, we lose.
When a new variant of influenza emerges, it starts with the viral equivalent of a poker hand made up of eight genetic segments. Like getting a four of diamonds or a queen of spades, H7N9 was effectively dealt a seven of Hemagluttinin (the H) and a nine of Neuraminadase (the N). Along with the genes on its other six segments, the H and N determine whether a new virus has the capability to spread in the global human population, the epidemiological equivalent of winning a championship pot.
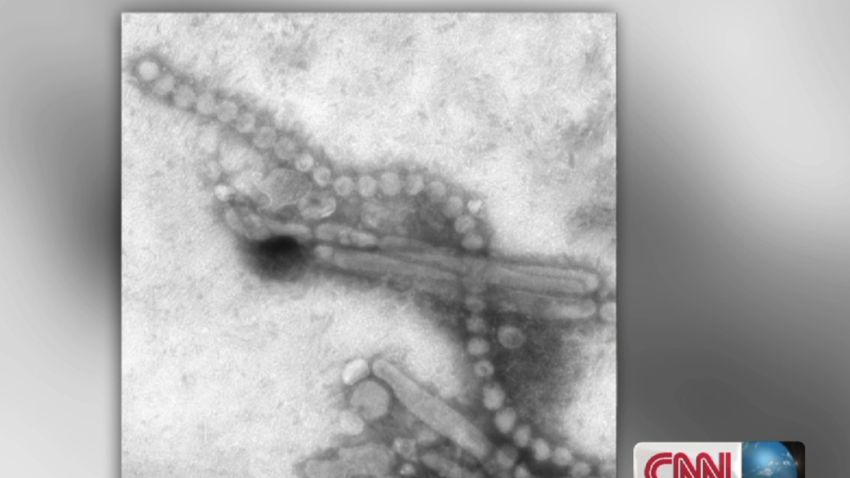
A new report today sheds light on the origins of H7N9. The virus, which jumps from chickens to humans, has killed around a third of the 134 known human cases since it was identified in April. H7N9 has also shown some capacity to spread from person to person. It has rightly put the global health community on alert.


Today’s report shows that H7N9 came about through repeated shuffling of influenza viruses present in wild birds and domestic ducks and chickens. It also documents the existence of a previously unidentified virus spreading in chickens.
The new virus, an H7N7, shares much in common with H7N9. And experiments on ferrets, the standard mammal model for influenza, suggest that like H7N9 it may have the capacity to infect humans. This means that undiscovered human cases of the H7N7 may exist, and with additional shuffles and further mutation this virus’s offspring could also represent a global health concern.
In today’s world the fight against epidemics like influenza overwhelmingly involves chasing the viruses that have already begun to spread in people. The argument is that given all of the potential viruses and mutations that could lead to the next big one, attempting to predict or prevent it is a fool’s errand. It’s increasingly clear that argument is wrong.
Influenza epidemics are anything but random. The origins of H7N9 and H7N7 tell a specific story of transmission from wild birds to ducks and eventually chickens and people.
Because chickens are so numerous – at 19 billion their numbers dwarf other domestic animals – and commonly sold live in some places, viruses that can spread efficiently in our favorite consumable bird increase their opportunity to make that fateful jump into people. And because wild birds, the ultimate origin of all influenza viruses, share close connections with domestic ducks, from whom the H and N genes jumped, these ducks become an important stepping stone for new viruses.
Even what’s absent in the viruses’ genes themselves tell us something useful. The N genes in both the new H7N9 and H7N7 viruses contain remarkably similar deletions of genetic material – modifications not seen in their duck virus ancestors. The independent occurrence of such deletions strongly suggests that the deleted region is critical to proliferation in chickens.
While still emerging, the story of H7N9 and H7N7 suggest highly practical recommendations. Ducks could help create the next pandemic influenza, so monitor them closely and consider ways to keep them separate from wild birds and other domestic animals.
As humans working alongside domestic birds will likely be hit first, monitor these sentinel populations, particularly in regions with novel types of avian influenza. Track the genetic geography of influenza during the process of its emergence as it moves between locations and wild and domestic animal hosts, because some genes will have a higher chance of successfully spilling over.
Together these steps will permit us to monitor the viruses we have most to fear from and optimize control measures, like the usefully deployed, but costly, closure of poultry markets.
Increasingly these recommendations are being heeded. The study released today represents part of a larger global effort to take stock of the viruses that can infect us, to document where they reside and to catch them when they spill over.
Governments, academic institutions, nonprofits and even a fledgling but rapidly growing private sector are working together in an unprecedented way to address a problem that increasingly threatens our globally interconnected world.
Together with our Chinese colleagues at the Guangdong Institute of Public Health, for example, we work to monitor new influenza and other viruses among people working in markets and other environments in precisely the regions where these viruses are emerging. The work represents a partnership with USAID’s Emerging Pandemic Threats Predict program, which supports these and other similar activities throughout the viral hotspots of the world.
The work is just beginning. Together with novel digital tools like monitoring internet search patterns and social media for signs of new epidemics, we are adding to a growing arsenal of approaches to catch epidemics and stop them before they become pandemics. In an increasingly interconnected world, where new epidemic threats surround us, it is time we elevate prediction and prevention of epidemics to a central element of global health.
A poker player may not be able to predict exactly when a royal flush will appear, but whoever is counting the cards and tracking the hands will always win in the end.
Follow us on Twitter @CNNOpinion.
Join us on Facebook/CNNOpinion.
The opinions expressed in this commentary are solely those of the authors.